Lustman et al., (2000) ‘Fluoxetine for depression in diabetes: a randomized double-blind placebo controlled trial’, Diabetes Care 23 (5), 618–23);
Background
This is the second study we will be looking at from the ‘Features of adherence to medical regimes‘ section of ‘Healthy Living’, as part of your OCR A2 Health and Clinical Psychology course. It is further categorised into ‘Measuring non-adherence‘
Lustman et al (2000) studies measuring non-adherence, there are several ways that non-adherence can be measured:
- Self-reports
- Therapeutic outcome
- Health worker estimates
- Pill & bottle counts
- Mechanical methods
- Biochemical tests
However, there are several problems with measuring adherence to medical regimes. Firstly, if it is a self-report, there is nothing to stop a patient lying. Secondly, pill and bottle counts may not find non-adherence, but incorrect usage and it would be very difficult to tell the difference without a self-report, which of course has it’s own problems. Biochemical tests, such as blood tests may also be flawed because they can test for drugs, but they cannot determine the frequency and consistency with which the patient is using the medical. One of the better ways to measure adherence to medical regimes is to observe them, however this could lead to demand characteristics which would be not present without the observer and thus become an invalid measurement.
Synopsis
Lustman et al (2000) – measured adherence in their research into diabetes and depression. Major depression is present in 15-20% of people with type I and II diabetes.
Aim
To assess the efficacy of the anti-depressant Fluoxetine in treating depression by measuring glycemic control.
Method and Design
A randomised control double-blind study.
A double-blind study means that both the researcher and the participants are unaware if the patients are taking the medication or a placebo, an external invigilator usually will know, but will not be able to affect the results of the study.
Participants
60 participants with diabetes.
26 had type I diabetes
34 had type II diabetes
Procedure
Patients randomly assigned to either the Fluoxetine or placebo group. This randomisation was completed by a computer algorithm.
The study was conducted over 8 weeks.
Patients were assessed for depression using psychometric tests and their adherence to their medical regimen was measured by measuring their GHb (gamma-Hydroxybutyric acid)
“The 8-week treatment period began with the
baseline visit and included subsequent visits
at the end of treatment weeks 3, 5, and
8. Fluoxetine dosing began at 20 mg a day
in the morning and could be increased to a
maximum of 40 mg daily in the morning
dependent upon side effects and clinical
response. Study outcomes were assessed at
baseline and at the conclusion of the 8-week
treatment period”
Lustman et al (2000)
Findings
Patients using Fluoxetine reported lower levels of depression. They also had lower levels of GHb, which indicated their improved adherence.
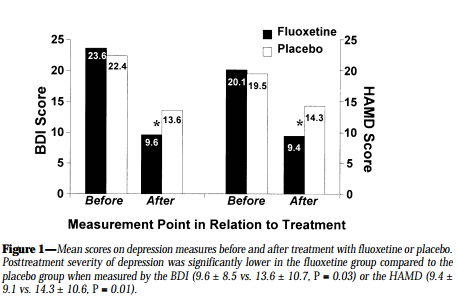
Conclusion
Measuring GHb in patients with diabetes indicates their level of adherence to prescribed medical regimes.
Greater adherence was shown by patients who were less depressed, and previous research has suggested that reducing depression may improve adherence in diabetic patients.
“Fluoxetine effectively reduces the severity of depression in diabetic
patients.”
-Lustman et al (2000)
Lustman et al (2000) Evaluation
+ Validity – the independent variable was well defined and manipulated and therefore we can argue that the study was high in validity
– Ethnocentrism – the sample considered of only people with diabetes therefore we cannot easily generalise the results to other populations.
+ Scientific – using a double blind study is highly scientific as nor the experimenter nor can the participants confound upon the results.
+ Useful – the studies results and conclusions are useful for increasing the adherence of patiences.
References
Further Reading
Psych Yogi’s Top Ten Psychology Revision Tips for the A* Student